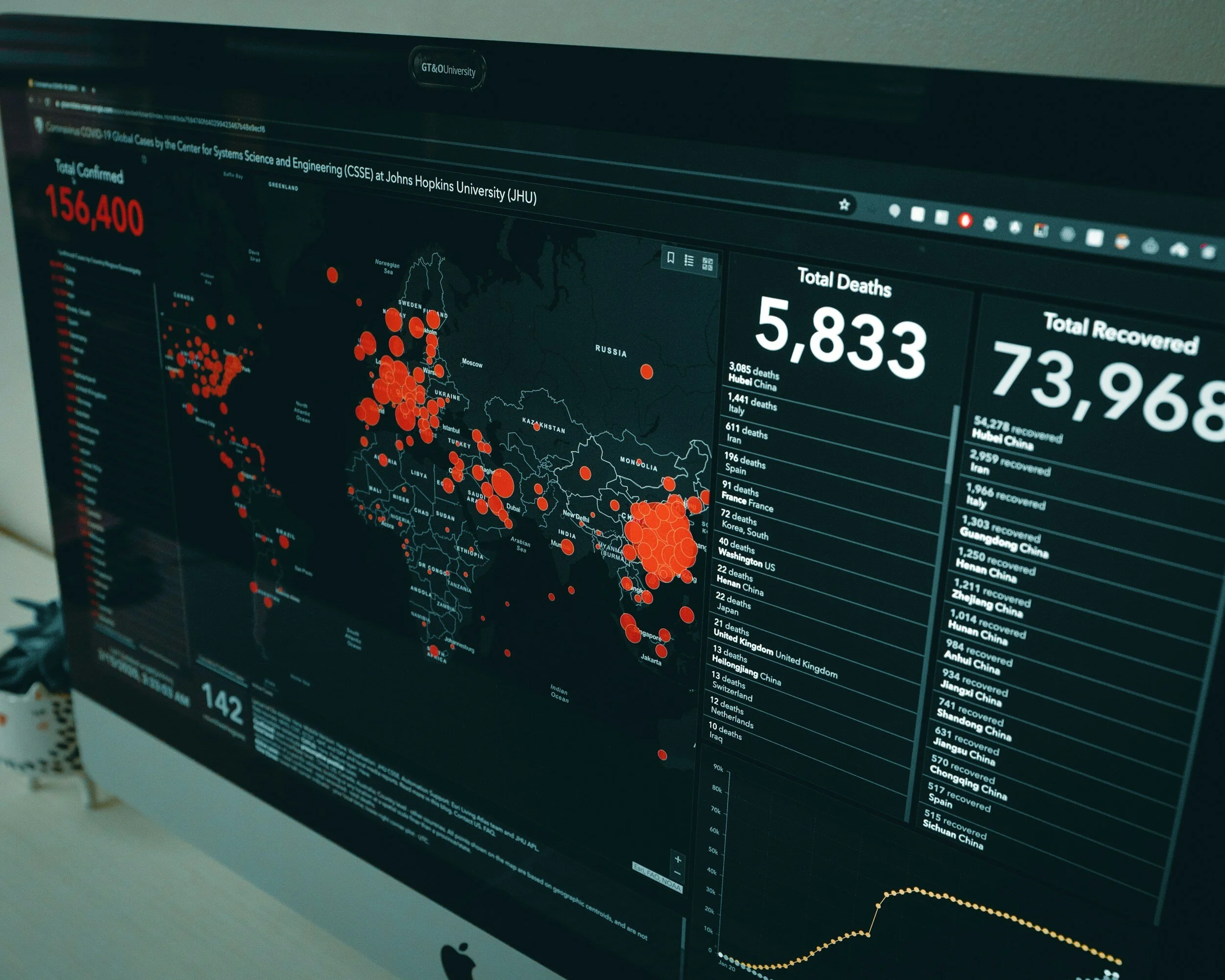
During the tumultuous days of the COVID-19 pandemic, Malawi's Ministry of Health exhibited remarkable foresight and agility by deploying digital tools to enhance its response efforts. Despite being one of the world's poorest countries, Malawi swiftly harnessed the power of technology to support the Public Health Institute of Malawi (PHIM) and the general population through the One Health Surveillance Platform (OHSP) and WhatsApp Chatbot tools, respectively. Notably, Malawi achieved a pioneering milestone by becoming the first African nation to deploy digital COVID-19 vaccination certificates.
In September 2023, Malawi received crucial funding from the Research Investment for Global Health Technology Foundation’s (RIGHT Foundation) "Evidence Generation Award (EGA)" to undertake the PAMODZI COVID-19 Study ("PAMODZI" meaning "together" in Chichewa, Malawi's official language). This national-level study employs a mixed-methods approach to assess the impact of OHSP and Chatbot tools during the country's COVID-19 response, shedding light on the effectiveness of digital solutions in strengthening healthcare infrastructure during the pandemic.
This study will go through five core stages:
Stage 1: Illuminating Digital Solutions and the OHSP Usage
The study commences with a comprehensive Delphi Study involving 40 expert panel participants, including representatives from the Ministry of Health (MOH), Public Health Information Management (PHIM), border health officials, and Integrated Disease Surveillance and Response (IDSR) stakeholders. This stage delves into Malawi's COVID response Digital Solutions/Services Architecture and the utilization of OHSP during the pandemic. It features face-to-face roundtable discussions and digital iterations via email to capture nuanced insights.
Stage 2: Engaging Health Service Providers and the General Population
Building on the insights from Stage 1, the study develops a national survey aimed at involving health service providers and the general population. This inclusive approach reaches out to various participants through patient and health advocacy groups across Malawi, with a total engagement target of 670 participants.
Stage 3: Unlocking Insights Through Retrospective Data Analysis
This stage focuses on a retrospective analysis of operational and service data collected by the Ministry of Health Chatbot tool. It encompasses diverse facets such as health education, risk communication, COVID-19 surveillance, vaccine-related information, and self-reporting data. Impressively, the Chatbot engaged with approximately 10,000 members of the Malawian community during the pandemic's peak.
Stage 4: Engaging Stakeholders Through a Collaborative Workshop
After data analysis, a two-day workshop brings together 40 panel members, representing the Ministry of Health and the general public. This unique gathering facilitates rigorous discussion and evaluation of the Chatbot tool's role in supporting Ministry of Health Officials and the public.
Stage 5: Informing Policy and Disseminating Findings
In the final stage, the study's findings culminate in a comprehensive report and policy recommendations. These insights are presented to the national-level Digital Health Technical Working Group within the Ministry of Health, guiding future strategies for digital health solutions in Malawi.
The PAMODZI COVID-19 Study represents a significant leap forward in understanding the impact of digital tools on healthcare responses during the COVID-19 pandemic in Malawi. Through stakeholder engagement, thorough assessments, and widespread dissemination of findings, this initiative paves the way for more effective healthcare solutions within the country and beyond.
This research is led by Luke International (LIN) in collaboration with the Malawi Ministry of Health (MoH), Public Health Institute of Malawi (PHIM), Mzuzu University (MZUNI), Malawi eHealth Research Center (MERC) and University College Cork (UCC).
Funding for this research is provided by the RIGHT Foundation.
Timeline: September 2023 to August 2024.